Precautionary Recall – Children’s Blackcurrant Cough Syrup
We are working with Bell, Sons & Co to recall own-brand blackcurrant cough syrup due to possible mould.

People should check bottles of some own-brand children’s blackcurrant cough syrup to see if they need a replacement.
A problem with one of the ingredients in these medicines has been identified which could cause them to allow mould to grow. There is a low risk that the mould could make the child unwell or cause a reaction, although to date we are not aware of any cases where this has happened. As a precaution it has been decided to recall affected batches.
Only a small number of batches from 8 different products are being recalled. No other cough syrups are affected.
Dr Sam Atkinson, the Medicines and Healthcare products Regulatory Agency’s (MHRA) Director of the Inspection, Enforcement and Standards Division said:
Check if you have any of the listed cough syrups and if you do, please don’t use them. Take them back to where you bought them from.
The mould is not always visible, so return any of the affected cough syrup bottles, even if it looks okay to use. “If your child has recently taken one of these cough syrups, and, in the unlikely event that they have become unwell or had a reaction, please speak to your GP, pharmacist or other healthcare professional.
Our highest priority is making sure the medicines you and your family take are safe. This is why, even though there is a low risk of a reaction, we have asked the company to carry out this recall and why we want people to check their medicine cabinets.
As with any medicine, we strongly encourage anyone to report any suspected side effects to us via our Yellow Card Scheme.
Notes to Editor
| Brand and Product Description | Batch Number(s) | Expiry Date |
| Asda Children’s Dry Cough Syrup Glycerol Blackcurrant Flavour | 274V1, 276V1, 278V1, 283W1 | 01-Aug-2020, 01-Oct-2020, 01-Dec-2020, 01-Feb-2021 |
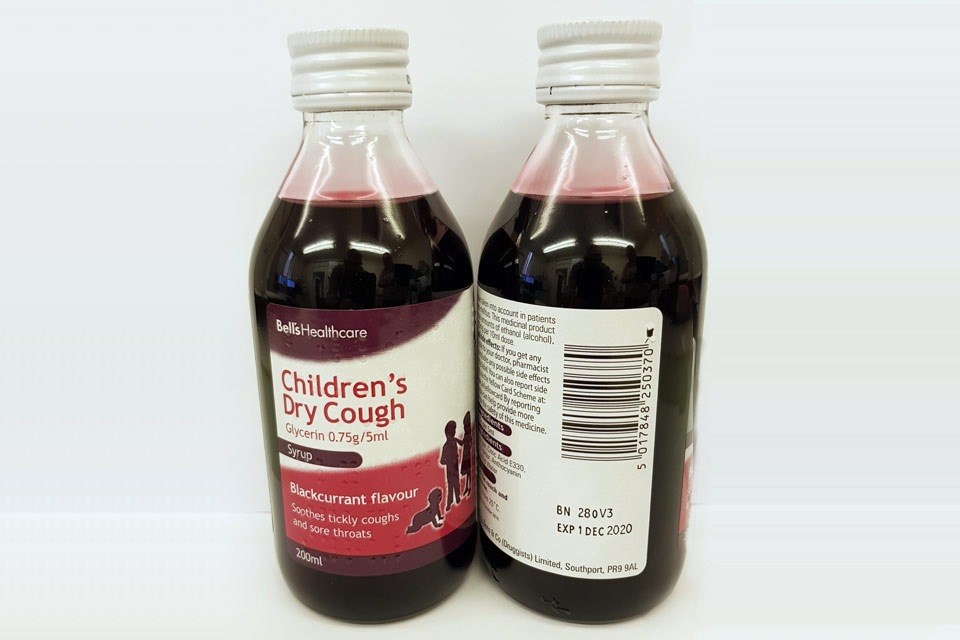
| Bell’s Healthcare Children’s Dry Cough Glycerin 0.75g/5ml Syrup | 280V3 | 01-Dec-2020 |
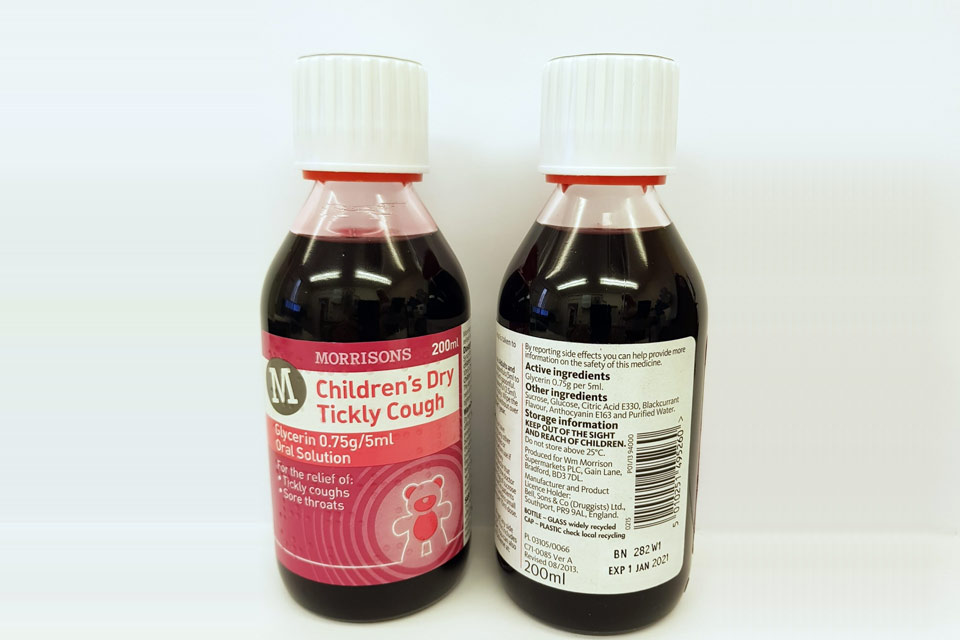
| Morrisons Children’s Dry Tickly Cough Glycerin 0.75g/5ml Oral Solution | 282W1 | 01-Jan-2021 |
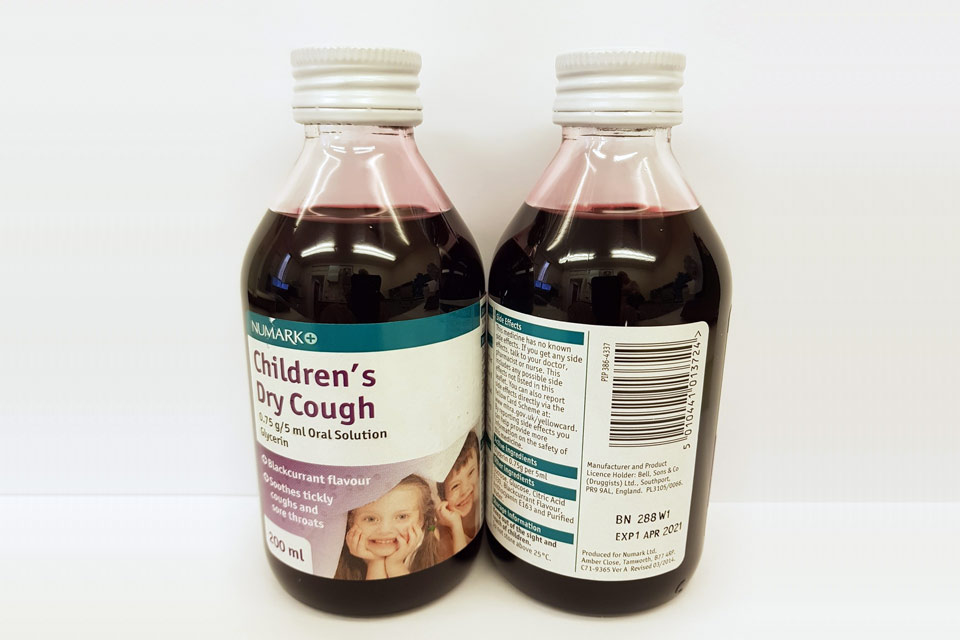
| Numark Children’s Dry Cough 0.75 g/5 ml Oral Solution | 280V1, 288W1 | 01-Dec-2020, 01-Apr-2021 |
| Sainsbury’s Children’s Dry Cough 0.75g/5ml Syrup | 275V1 | 01-Sep-2020 |
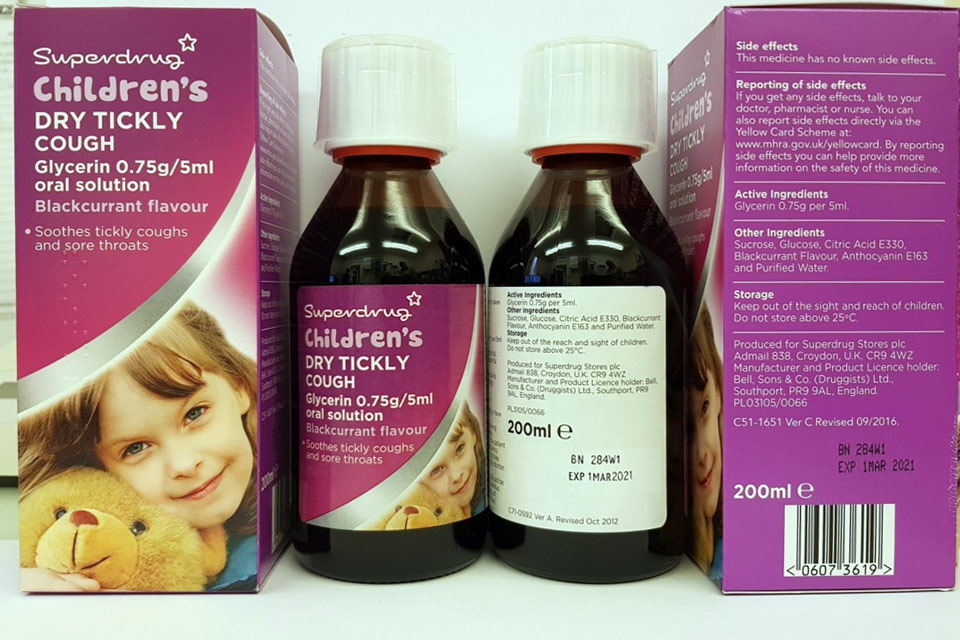
| Superdrug Children’s Dry Tickly Cough Glycerin 0.75 g/5 ml Oral Solution | 280V2, 284W1 | 01-Dec-2020, 01-Mar-2021 |
| Tesco Children’s Dry Cough Syrup | 277V1, 278V2, 281W1 | 01-Oct-2020, 01-Dec-2020, 01-Jan-2021 |
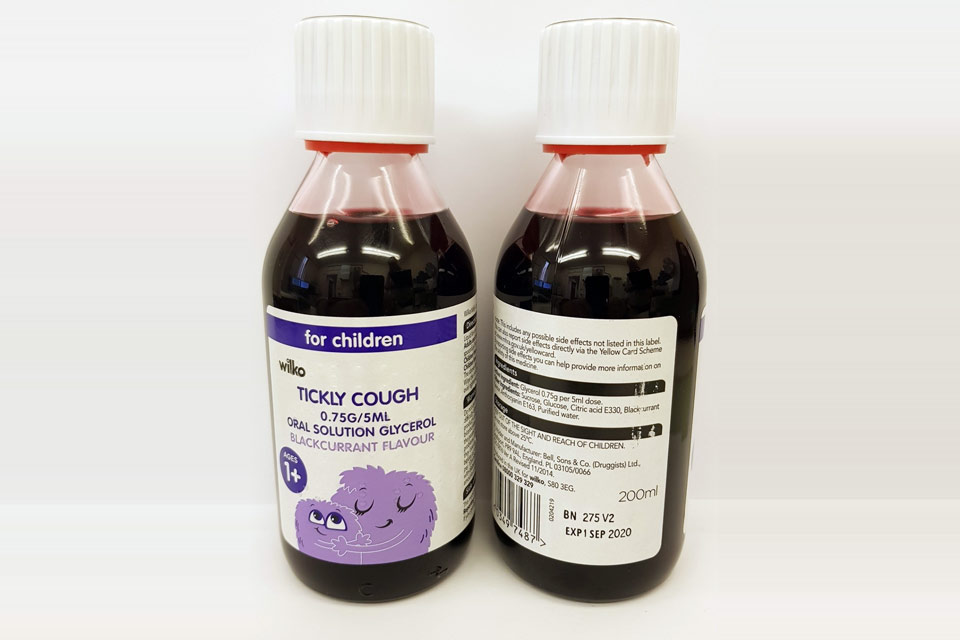
| Wilko Tickly Cough 0.75g/5ml Oral Solution | 275V2 | 01-Sep-2020 |
- MHRA is responsible for regulating all medicines and medical devices in the UK. All our work is underpinned by robust and fact-based judgments to ensure that the benefits justify any risks. MHRA is a centre of the Medicines and Healthcare products Regulatory Agency which also includes the National Institute for Biological Standards and Control (NIBSC) and the Clinical Practice Research Datalink (CPRD). The Agency is an executive agency of the Department of Health. www.mhra.gov.uk
- Link to Yellow Card Scheme
- Images: